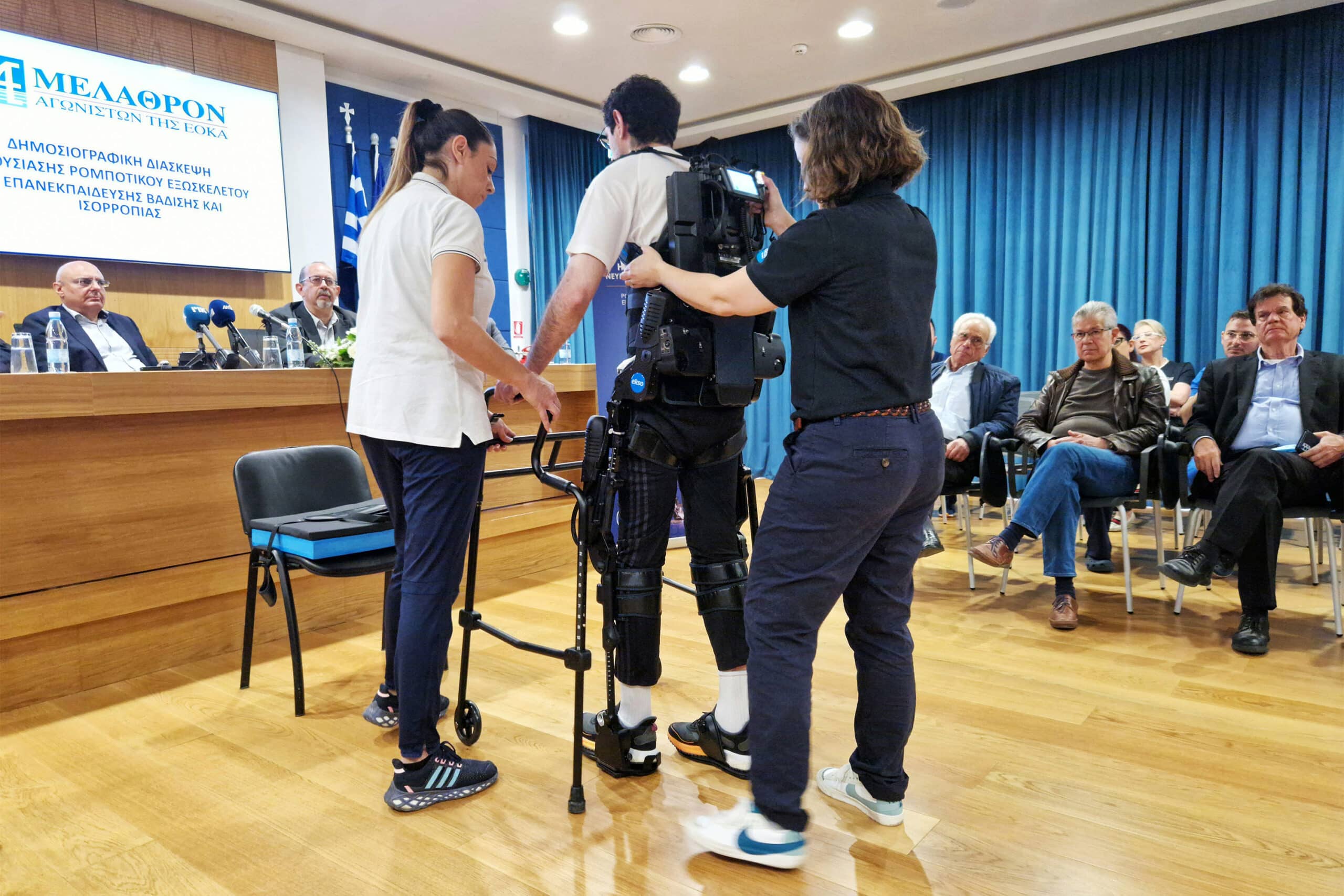
Medical centre unveils robotic exoskeleton
– A Limassol-based medical centre has acquired the first robotic exoskeleton in Cyprus.
– The medical centre, Melanthron Agoniston Eoka, invested over €200,000 for an EksoNR robotic exoskeleton.
– The health ministry covered half of the cost, €100,000, through a medical equipment subsidy scheme.
– Robotic exoskeletons are designed to support individuals with difficulty walking due to neurological conditions or injuries.
– EksoNR is FDA-cleared for use with patients suffering from acquired brain injuries (ABI), multiple sclerosis (MS), stroke, and spinal cord injuries (SCI).
– Not all patients can use robotic exoskeletons; there are specific criteria for eligibility, such as a maximum height of 1.95 meters and weight of 100 kilograms.
– This technology is new to Cyprus but there are approximately 256 similar exoskeletons in Europe and four in Greece.
– The health ministry expressed satisfaction with the acquisition of the robotic exoskeleton for the rehabilitation centre.